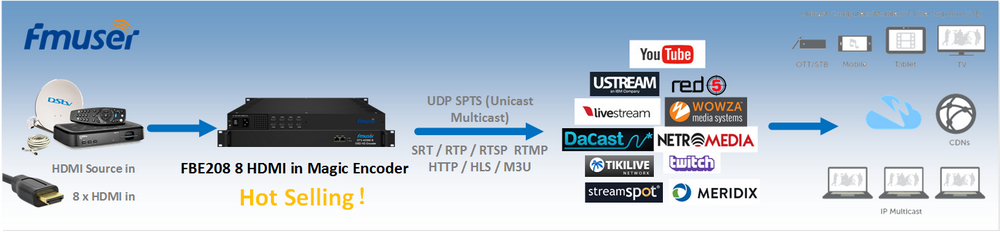
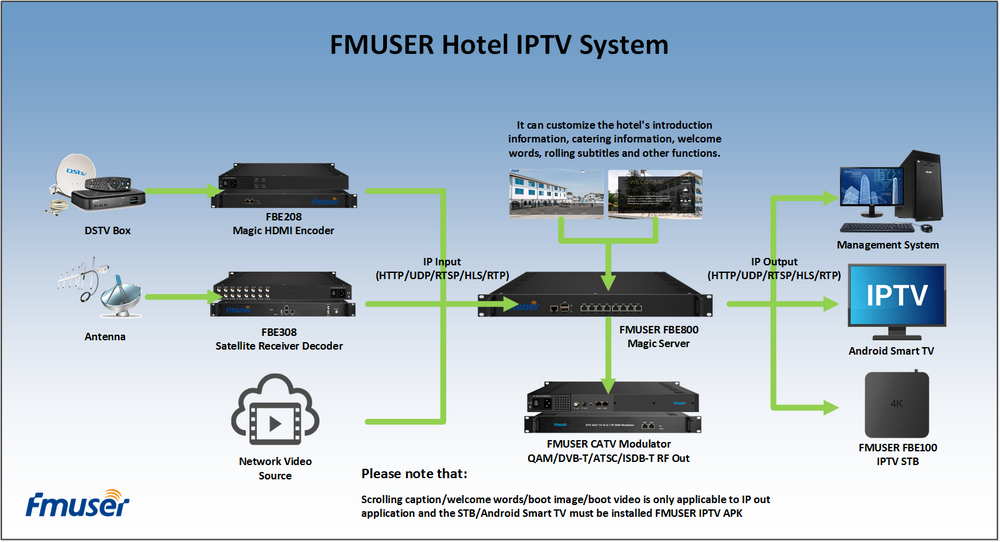
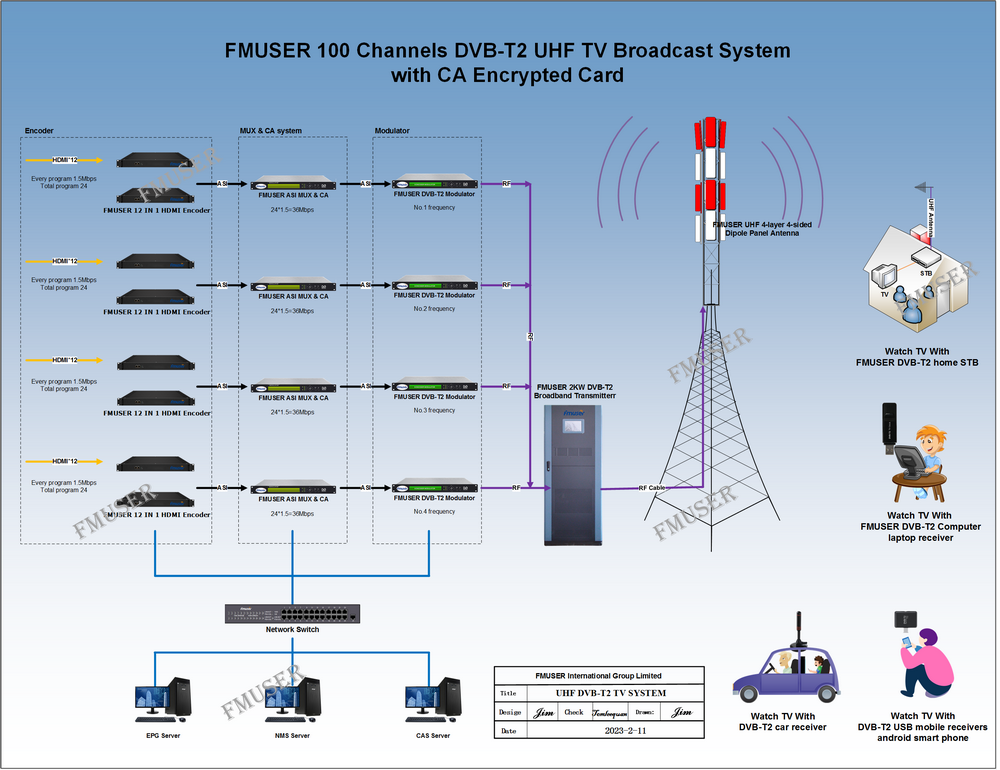
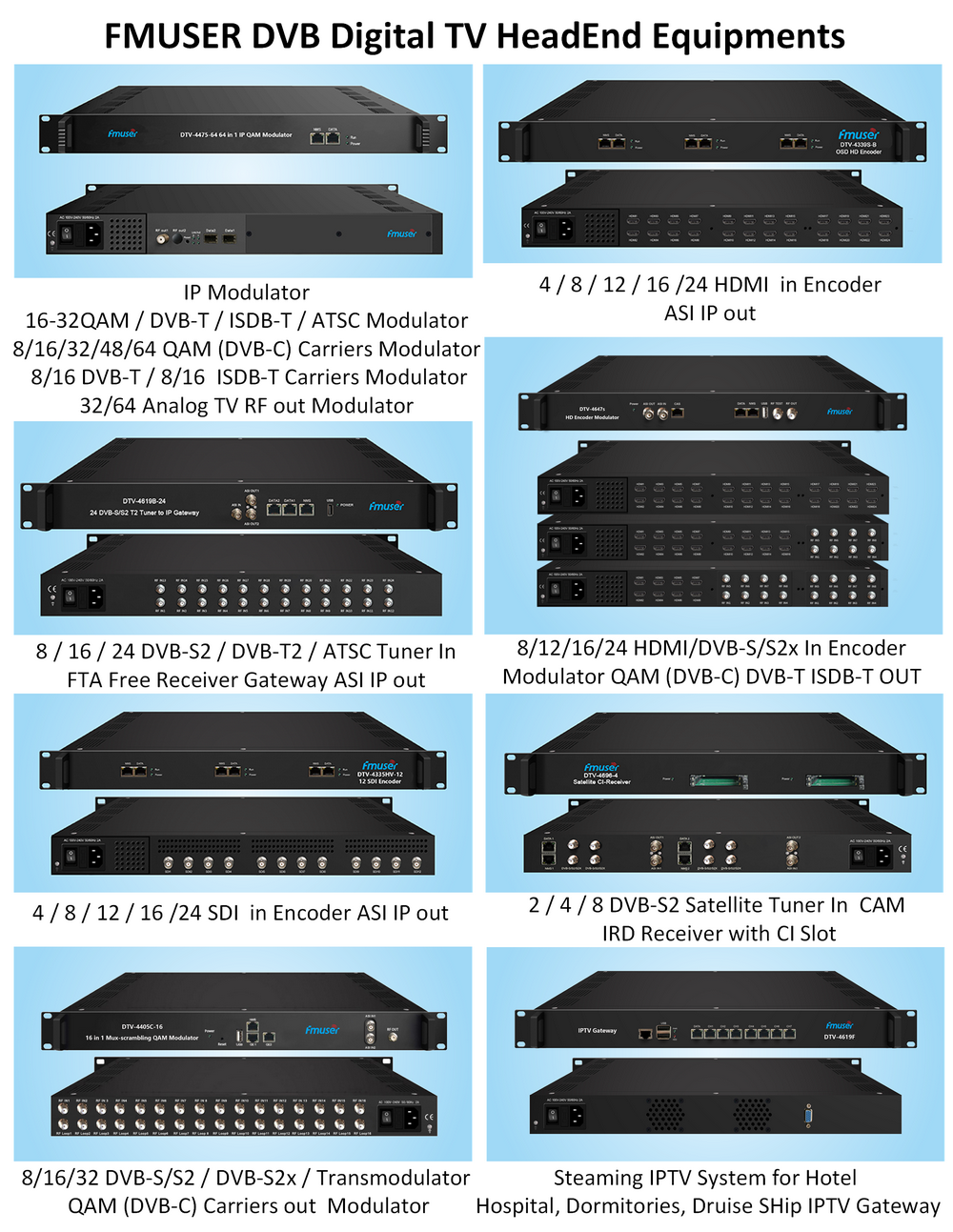
The main raw material: graphite powder (particle size less than 30μm particle content greater than 95%, a carbon content of 99.85%.), Concentrated sulfuric acid (95% -98%), potassium permanganate, sodium nitrate, 30% hydrogen peroxide, hydrochloric acid, barium chloride , 80% hydrazine hydrate was prepared graphite oxide (GO) of
Prepared method graphite oxide Hummers
Specific process: good fitted in an ice bath 250 mL reaction flask, an appropriate amount of concentrated sulfuric acid was added, the solid mixture was added with stirring 2 g of graphite powder and 1 g of sodium nitrate, and then added portionwise 6 g of potassium permanganate, controlling the reaction temperature did not exceed 20 ℃, the reaction was stirred for a period of time, then warmed to about 35 ℃, stirring was continued for 30 min, then slowly adding a certain amount of deionized water, 20 min after continued mixing, and adding an appropriate amount of hydrogen peroxide reduction of the residual oxidizing agent, The solution turned bright yellow. Filtered hot and washed with 5% HCl solution and deionized water until the filtrate was free of sulfate is detected. Finally, the filter cake was placed in a vacuum oven at 60 deg.] C sufficiently dried, stored for use.
Preparation of graphene
100 mg of the graphite oxide is dispersed in an aqueous solution of 100 g to give a tan suspension is then dispersed under ultrasonic irradiation for 1 h to give a stable dispersion. Then, in a four-mouth flask, heated to 80 ° C, add 2 mL of hydrated hydrazine, and then filtered after 24 h under this condition, and the resulting product was built multiple times with methanol and water, and then dried at 60 ° C The box is fully dry, and the spare is stored.
Specific experimental steps:
A: Preparation of graphene oxide
1: a large beaker 250Ml, which put ice cubes, ice-water bath provided
2: amount of test tubes 23mlH2SO4, then graphite electronic balance weigh 1g, 0.5g sodium nitrate, 3g of potassium permanganate
3: tweezers companies have been switched into the conical flask, then gently poured into the conical flask of concentrated sulfuric acid, and then placed in a magnetic stirrer.
4: graphite was added and mixed sodium Erlenmeyer flask, and the reaction was stirred for three minutes, and then potassium permanganate is added flasks
5: controlling the temperature less than 20 ℃, the reaction was stirred for 2 hours
6: Heat to 35 ℃, stirring was continued for 30 minutes
7: The configuration 46mL distilled water and deionized water (14 ° C)
8 to 30 minutes after the reaction, deionized water was added to the conical flask, and then the temperature was raised to 98 deg.] C, heating was continued for 20min, the solution was brown, red smoke smoke
9: Remove 5g hydrogen peroxide (30%), the conical flask was added
10: Remove the conical flask was filtered while hot, and washed with deionized water, and HCL, until the remaining solid in the filter is stable, the paper taken out with tweezers, and then a clean filter paper in the bottom of the liner, into a dried deg.] C to 60 box sufficiently dried.
Preparation of graphene
1: The dried graphene oxide, dispersed in 100g 100mg removed aqueous solution to give a brown yellow suspension
2: The suspension is placed in an ultrasonic cleaning tank, the ultrasonic dispersion for 1 hour
3: Remove the solution into four beaker, heated to 80 ℃, 20ml of hydrazine hydrate was added dropwise, the reaction was filtered 24 hours
4: The product thus obtained was washed with methanol and water
5: obtained solid was sufficiently dried in an oven at 60 ℃, stored for use
In experimental raw materials
Concentrated sulfuric acid: strong protic acids, into the graphite interlayer. Potassium permanganate: Strong oxidants, to generate graphite oxide (GO) through ultrasonic peeling graphene oxide. Hydrazine hydrate: reducing, out of the graphene oxide surface oxygen functional groups, to obtain graphene. Sodium nitrate: Under strong acids, with strong oxidizing nitrate. Hydrogen peroxide: removing excess potassium permanganate oxidation, oxidation of divalent manganese ions are removed. Dilute hydrochloric acid: wherein wash metal ion, sulfate ion, barium chloride: wherein sulfate ion detection.
Improved Preparation of graphite oxide Hummers Method
Improved Preparation of graphite oxide Hummers Method: good fitted in an ice bath 500 ml reaction flask, the graphite powder and 5 g 5 g sodium nitrate was mixed with 200 ml of concentrated sulfuric acid uniformly, added with stirring 25 g of potassium perchlorate, evenly, and then was added in 15 g of potassium permanganate, the control temperature does not exceed 20 ℃, after a period of stirring, the ice bath removed, the reaction flask was transferred to a magnetic stirrer, electromagnetic stirring was continued for 24 h. Thereafter, slowly with stirring 200 ml of deionized water, the temperature rises to about 98 deg.] C, stirring for 20 min, an appropriate amount of added hydrogen peroxide reducing residual oxidant, and the solution turned bright yellow. Graded graphite oxide is then separated from the suspension was centrifuged at 10000 rpm, and successively washed with 5% HCl solution and deionized water were separated until pH = 7. The resulting filter cake was dried in vacuo to obtain the graphite oxide. Preparation of graphite oxide process shown in Figure 3-1.
Be
Note: the reaction low temperature (<20 ℃),="" since="" the="" temperature="" is="" low,="" oxidation="" of="" sulfuric="" acid="" is="" relatively="" low,="" the="" driving="" force="" is="" insufficient="" to="" provide="" the="" intercalation="" reaction,="" therefore,="" is="" not="" the="" original="" graphene="" oxide.="" after="" addition="" of="" potassium="" permanganate="" oxidizing="" solution="" enhancing="" edges="" of="" graphene="" it="" is="" first="" oxidized.="" with="" the="" increase="" of="" the="" amount="" of="" potassium="" permanganate,="" and="" for="" the="" oxidation="" process,="" the="" carbon="" atoms="" in="" the="" graphite="" planar="" structure="" becoming="" planar="" molecules="" with="" a="" positive="" charge,="" due="" to="" oxidation="" edge="" portion="" curling.="" in="" this="" case,="" hydrogen="" sulfate="" and="" sulfuric="" acid="" molecules="" gradually="" into="" the="" graphite="" interlayer,="" forming="" sulfuric="" acid="" -="" graphite="" intercalation="" compound.="" when="" the="" temperature="" of="" the="" reaction=""><40 ℃),="" sulfuric="" acid="" -="" graphite="" intercalation="" compounds="" are="" deep="" oxidation,="" brownish="" mixture.="" the="" reaction="" temperature="" (90="" ℃="" -100="" ℃)="" stage,="" the="" residual="" concentrated="" sulfuric="" acid="" with="" water="" to="" effect="" evolution="" of="" considerable="" heat,="" so="" that="" the="" temperature="" of="" the="" mixture="" rose="" to="" about="" 98="" deg.]="" c,="" sulfuric="" acid="" -="" hydrolysis="" graphite="" intercalation="" compounds,="" a="" large="" amount="" of="" water="" into="" the="" sulfuric="" acid="" -="" a="" graphite="" layer="" the="" interlayer="" compound,="" interlayer="" water="" becomes="" parallel="" extrusion="" sulfate,="" water="" and="" hydrogen="" sulfate="" ion="" and="" oh-="" ion="" exchange="" occurs,="" replacement="" of="" part="" of="" the="" hydrogen="" sulfate="" ion="" and="" in="" combination="" with="" the="" carbon="" atom="" on="" the="" level="" of="" the="" graphite,="" resulting="" in="" graphite="" interlayer="" spacing="" becomes="" large,="" the="" volume="" expansion="" phenomenon="" occurs="" graphene,="" bright="" yellow="" solution="" was="" formed.="" in="" the="" washing="" and="" drying="" process,="" an="" oh="" and="" h="" +="" between="" the="" graphite="" oxide="" layers="" bound="" off="" in="" the="" form="" of="" water="" molecules,="" thus="" becoming="" the="" product="" by="" staphylococcus="" black.="" preparation="" graphene:="" figure="" 3-2="" is="" prepared="" graphene="" from="" graphite="" oxide="" process="" flow="" diagram.="" crush="" graphite="" oxide,="" weighed="" 300="" mg="" dispersed="" in="" 60="" ml="" of="" deionized="" water="" to="" give="" a="" tan="" suspension,="" 1="" h="" after="" ultrasonic="" dispersion="" to="" obtain="" a="" stable="" colloidal="" suspension.="" then="" transferred="" to="" a="" four-necked="" flask,="" was="" added="" 600="" mg="" of="" sodium="" borohydride="" and="" 50="" mg="" of="" sodium="" dodecylbenzenesulfonate,="" was="" heated="" to="" 80="" ℃,="" centrifuged="" at="" reflux="" for="" 16="" h,="" then="" under="" this="" condition,="" washed="" with="" acetone="" and="" deionized="" water="" to="" ph="7," the="" resulting="" cake="" was="" dried="" in="" vacuo="" to="" save="" backup,="" denoted="" gs1.="" prepared="" in="" the="" same="" manner="" under="" the="" conditions="" of="" a="" graphene="" gs="" without="" dispersant.="" be="" note:="" after="" graphite="" graphite="" oxide="" obtained="" strong="" oxidizing="" agent="" to="" form="" a="" hydroxyl="" group,="" an="" epoxy="" group="" and="" a="" carboxyl="" group="" at="" the="" six="" membered="" ring="" of="" the="" graphite="" layer.="" in="" one="" aspect,="" oxygen-containing="" group="" to="" improve="" hydrophilic,="" they="" are="" water-soluble="" introduced="" graphene,="" graphite="" oxide="" is="" increased="" in="" solubility="" in="" water,="" increase="" the="" stability,="" in="" this="" research,="" the="" multi="" it="" is="" used="" to="" prepare="" modified="" graphite="" ene.="" on="" the="" other="" hand,="" the="" introduction="" of="" oxygen-containing="" groups="" due="" to="" steric="" hindrance="" of="" the="" graphite="" interlayer="" spacing="" becomes="" large,="" reducing="" the="" agglomeration="" between="" the="" graphite="" layers.="" ,="" reading="" the="" full="" text,="" the="" technology="" area="" 2018="" chinese="" provinces="" graphene="" base="" summary="" novel="" nanomaterials="" graphene="" mimic="" nose="" dogs="" gas="" detection="" graphene="" domestic="" industry="" of="" false="" prosperity="" ushered="" in="" the="" form="" of="" sino-us="" differences="" reflect="" what="" are="" the="" most="" environmentally="" friendly="" materials="" _promising="" new="" materials="" introduced="" read="" an="" article="" new="" situation="" and="" development="" trend="" of="" environmentally="" friendly="" materials="">
Our other product: